Abstract
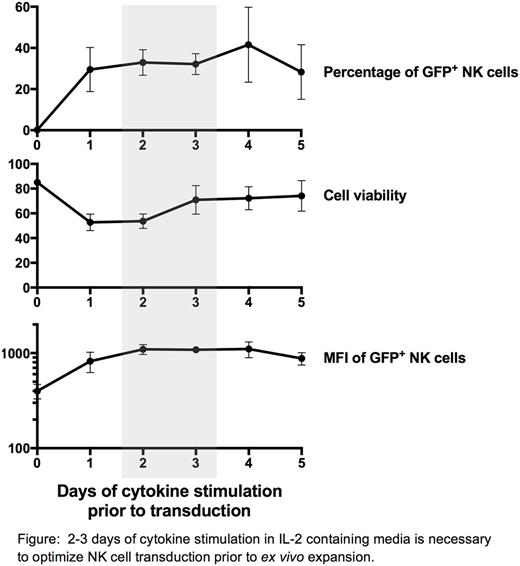
Natural killer (NK) cells have the unique capacity to lyse tumor cells without the need for antigen specific receptors. Modulation of NK cell cytokine, chemokine, and activating and inhibitory receptor expression using gene regimens represents an attractive strategy to further bolster NK cell anti-tumor activity for the purpose of NK cell immunotherapy. Current efforts in clinical-grade genetic modification of patient-derived cells, used in immunotherapy protocols, rely on stable transduction mediated by viral vectors. However, primary NK cells are notoriously difficult to transduce using viral vectors. Here we describe a lentiviral vector-based approach that results in an efficient, robust, and highly reproducible method of stable gene transfer into primary human peripheral blood-derived NK cells, which can subsequently be expanded ex vivo for adoptive infusion into humans. In vitro experiments established highly efficient transduction of ex vivo expanded NK cells could be achieved by activation of primary NK cells in vitro for 2-4 days in cytokine containing media followed by transduction of activated NK cells using LV vectors 2-3 days before integration into our existing ex vivo expansion protocol using irradiated EBV-LCL feeder cells. Unless NK cells were first activated in cytokine-containing media, LV transduction was unsuccessful. Cytokine activation in media containing IL-2 for 2-4 days was found to be sufficient to achieve high transduction efficiency, while additional supplementation of the media with IL-15 or IL-21 had negligible effects. NK cell transduction efficiency and viability with the use of RetroNectin® was uniformly superior to polycation-based transduction protocols. In experiments where NK cells were transduced with identical lentiviral constructs with 8 different promoter sequences driving expression of EGFP, PGK, EFS, and SV40 promoters consistently demonstrated the highest transduction efficiencies reproducibly in the range of 25-60%. Remarkably, when transduced NK cells were stimulated ex vivo using a clinical grade irradiated EBV-LCL feeder cell line, 100-1000 fold expansions of transduced NK cells could be achieved with sustained transgene expression over two weeks. Transduced and expanded primary NK cells remained highly cytotoxic against K562 tumor targets without developing functional deficiencies in degranulation, IFNγ, or TNFα production. These data establish a simple method to produce large numbers (>1011) of genetically modified clinical grade NK cells suitable for infusion. The protocol described herein overcomes previous difficulties described with common transduction techniques opening new avenues to investigate the impact of adoptive infusion of primary NK cells stably transduced to express a variety of different transgenes of interest.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.